Atomic Mass Facts For Kids
Atomic mass is a measurement that reflects the average mass of an element's isotopes in atomic mass units, impacting various chemical properties and behaviors.
Set reading age
View for Kids
Easy to read and understand
View for Students
Clear, detailed explanations
View for Scholars
Deep dives and big ideas
Introduction
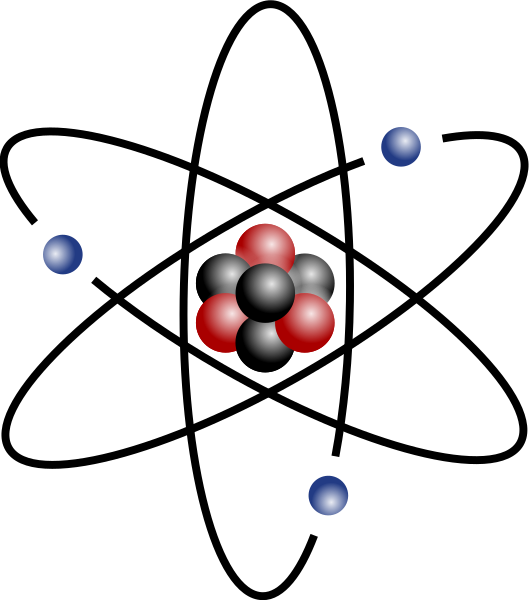
Atomic mass is an important idea in chemistry! 🧪It tells us how heavy an atom is compared to others. Atoms are tiny building blocks that make up everything around us—like trees, animals, and even our own bodies! The atomic mass helps scientists understand how atoms behave and interact with each other. Every element in the periodic table, like Hydrogen (H) and Oxygen (O), has its own atomic mass. For example, Hydrogen has an atomic mass of about 1 amu, while Oxygen is about 16 amu. Understanding atomic mass helps us unlock the secrets of the universe! 🌌
Gallery of Atomic Mass Facts For Kids
What Is Atomic Mass?
Atomic mass is the weight of an atom, measured in atomic mass units (amu). 📏Each atom contains protons and neutrons in its nucleus, which contribute to its mass. Electrons are tiny and don't add much weight. The atomic mass is like a number that tells us how heavy an element is. For instance, Carbon (C) has an atomic mass of about 12 amu. 📊When we find the atomic mass, we often round off to a whole number, but scientists use decimal points for accuracy. This helps with experiments and calculations in science! ⚗️
Atomic Mass Units (amu)
Atomic mass units, abbreviated as amu, are small measurement units! 📏One amu is equal to 1.66 × 10⁻²⁷ kilograms. That’s really tiny! Atoms are so small that we need this special unit to measure them. The amu helps scientists compare the weights of different atoms. For example, a Chlorine atom has an atomic mass of about 35.5 amu. 🔬This means it's heavier than other atoms like Helium, which has an atomic mass of about 4 amu. By using amu, scientists can better understand the importance of atomic masses in chemistry and the elements’ properties! 🌌
Calculating Atomic Mass
Calculating atomic mass is relatively simple! To find the atomic mass of an element, we take the number of protons and neutrons in the nucleus. 🧮Protons have a mass of about 1 amu, just like neutrons. A Carbon atom has 6 protons and 6 neutrons, so its mass is 12 amu! There are also isotopes, which are versions of an atom with different numbers of neutrons. For example, Carbon-14 has 8 neutrons, giving it a higher atomic mass of 14 amu! ⚖️ Scientists can use both basic math and special tools to make these calculations, helping them understand different elements!
Future Of Atomic Mass Research
The future of atomic mass research is bright! Scientists are always discovering new elements and isotopes, which helps us learn even more! 🌟They are exploring the nanoworld, where understanding atomic mass reveals secrets about materials and energy. Researchers are also studying atoms in extreme conditions like stars, which can teach us about the universe! 🌌With advancements in technology, we may develop new ways to measure atomic mass faster and more accurately! 🔬The more we understand about atomic mass, the more we can use this knowledge to create innovative solutions for challenges in health, environment, and technology! ⚙️
History Of Atomic Mass Measurement
The idea of atomic mass dates back to the 1800s! 📅John Dalton, an English scientist, was one of the first to study atomic theory around 1803. He used hydrogen as a reference, giving it an atomic mass of 1 amu. In 1869, Dmitri Mendeleev created the periodic table to help organize elements and their masses! 🌍Over time, scientists developed more accurate ways to measure atomic mass with tools like mass spectrometers. These tools are like super-advanced scales that help identify the weight of different atoms very precisely. 🔬
Importance Of Atomic Mass In Chemistry
Atomic mass plays a key role in understanding chemical reactions! ⚗️ When atoms combine to form molecules, knowing their atomic masses helps scientists figure out how much of each element is needed. For example, if we want to make water (H₂O), we need 2 hydrogen atoms and 1 oxygen atom, which totals to 18 amu! This information helps chemists create new materials, medicines, and even food! 🍽️ Atomic mass is essential for scientists to communicate accurately and to work on exciting experiments to discover new things in the world! 🌟
Applications Of Atomic Mass In Real Life
Atomic mass has many exciting real-life applications! 🌍In medicine, doctors use atomic mass to create medicines and treatments. For example, certain cancer treatments use radioactive isotopes, which are special versions of atoms with atomic mass that helps doctors target sick cells! 🏥In cooking, understanding atomic mass helps chefs know how to combine ingredients for perfect recipes! 🍳Atomic mass also plays a role in environmental science to study pollutants and their effects. By understanding atomic mass, scientists can solve problems and improve our world every day! 🔧🌱
Differences Between Atomic Mass And Molecular Mass
Atomic mass and molecular mass might sound similar, but they are different! 🌠Atomic mass is the mass of a single atom, while molecular mass is the total mass of a molecule, which is made up of two or more atoms. For example, a water molecule (H₂O) has one oxygen atom (16 amu) and two hydrogen atoms (1 amu each). So, the molecular mass of water is 18 amu! 💧Atomic mass is like looking at one piece of a puzzle, while molecular mass is about the whole puzzle completed with multiple pieces! Knowing both is important for understanding big chemical reactions! ⚡️
Atomic Mass Facts For Kids Quiz


Make things. Learn new skills. Share safely.
DIY is a creative community where kids draw, build, explore ideas, and share.
No credit card required