Alpha Particle Facts For Kids
Alpha particles are tiny particles made of two protons and two neutrons, identical to a helium nucleus, that play important roles in nuclear physics and medicine.

Set reading age
View for Kids
Easy to read and understand
View for Students
Clear, detailed explanations
View for Scholars
Deep dives and big ideas
Introduction
Alpha particles are special tiny particles found in the world of atoms! 🌌They are made up of two protons and two neutrons, which means they are like little bundles of positive energy! These particles are the same as helium nuclei, which is what makes balloons float. 🎈Scientists discovered alpha particles a long time ago, and they help us understand the building blocks of everything around us! From the stars in the sky to the air we breathe, alpha particles play a big role in our universe. They can even be detected using special tools. Let's learn more about them!
Gallery of Alpha Particle Facts For Kids
Alpha Decay Process
Alpha decay is the process by which unstable atoms release alpha particles! 🌟This happens when an atom is too heavy and needs to lose some weight! When an atom undergoes alpha decay, it releases an alpha particle, which is made of two protons and two neutrons. After losing this alpha particle, the atom becomes a different element! For example, when radium-226 decays, it turns into radon-222. This process is slow and can take years! ⏰Scientists watch for these changes to learn about the ages of rocks and fossils, too!
Alpha Particle Emission
When certain unstable atoms, like uranium, break apart, they release alpha particles. This process is called alpha particle emission. 🌪️ During this process, the atom loses two protons and two neutrons, turning into a new element! For example, when uranium-238 releases an alpha particle, it becomes thorium-234. Each time this happens, it helps scientists understand how elements change over time! ⏳Although alpha particles can’t travel through air very far, they can be dangerous if they enter the body. That's why safety is super important!
Detection Of Alpha Particles
Detecting alpha particles is essential for scientists to study them! 🚨Special devices called alpha particle detectors are used for this. One common type is the Geiger-Müller Counter, which clicks when it detects radiation! 🔊Other detectors use materials like films that change color when alpha particles hit them. Scientists also use scintillation counters that flash when particles interact with special crystals. The detection of alpha particles helps keep people safe from radiation and makes sure that scientists understand their environment better! It’s like a big game of hide and seek with tiny particles! 🔍
Discovery Of Alpha Particles
In 1899, a scientist named Ernest Rutherford made an important discovery! 🤓He found that certain materials, like uranium, gave off mysterious rays. He called these rays “alpha rays.” A few years later, he realized that these rays were actually made up of alpha particles! Rutherford's work helped us understand radioactivity and how different particles behave. 🧪He is considered one of the fathers of nuclear physics! His discoveries led to many important scientific advances, including how we use radiation today in medicine and energy.
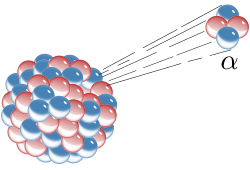
Structure Of Alpha Particles
An alpha particle is like a mini version of a helium atom! 🐣It contains two protons, which have a positive charge, and two neutrons that have no charge at all. This combination gives the alpha particle a total charge of +2. This means it can be attracted to negatively charged particles, like electrons! 🌟Alpha particles are quite heavy compared to other particles. They move slower than beta particles and gamma rays, but they have a lot of energy packed inside. This structure helps them do incredible things, like cause reactions!
Alpha Particles In Nuclear Physics
Alpha particles are super important in nuclear physics! 🔬Scientists study them to understand the forces that hold atoms together. These particles help explain concepts like radioactivity and nuclear decay. Also, they help researchers learn how elements turn into each other through a process called transmutation. 🌈Understanding alpha particles has led to many discoveries about the atomic nucleus and energy! Moreover, learning about them can help us find new ways to create clean energy using nuclear reactors, making the world a better place!
Historical Importance In Atomic Theory
The discovery of alpha particles played a big role in shaping our understanding of atomic theory! 🌎Scientists like Ernest Rutherford and Niels Bohr helped change how we view atoms. They showed us that atoms are made of smaller parts and that they can emit particles! This discovery opened up a new world of physics, helping us understand everything from chemicals to the universe itself! 🌌Today, atomic theory is crucial for many fields, such as chemistry and engineering. By learning about alpha particles and their history, we get to understand how science has evolved over time! 📚
Comparison With Beta And Gamma Radiation
Alpha particles are not the only kind of radiation! There are also beta particles and gamma rays! 😊Alpha particles are big, heavy, and positively charged. They don’t travel far and can be stopped by paper or the skin. In contrast, beta particles are lighter and can travel further, being stopped by plastic or aluminum. Gamma rays, however, are super tiny and can go through almost anything, including humans! 💡Understanding these differences helps scientists know how to protect us from harmful radiation and use it for good things, like medicine!
Applications Of Alpha Particles In Medicine
Alpha particles have some very cool uses in medicine! 🩺Doctors use them in a process called radiation therapy to help kill cancer cells. When cancer cells are treated with alpha particles, they get damaged, and this can stop them from growing and spreading! ⚗️ Scientists are also researching how to use alpha particles to make special medicines that can target diseases more effectively. This means they can help send medicine directly to the unhealthy cells, which is super smart and effective! Medical research with alpha particles is helping save lives every day!
Alpha Particle Facts For Kids Quiz


Make things. Learn new skills. Share safely.
DIY is a creative community where kids draw, build, explore ideas, and share.
No credit card required