Alpha Decay Facts For Kids
Alpha decay is a type of radioactive decay in which an unstable atomic nucleus emits an alpha particle, resulting in a new element.

Set reading age
View for Kids
Easy to read and understand
View for Students
Clear, detailed explanations
View for Scholars
Deep dives and big ideas
Introduction
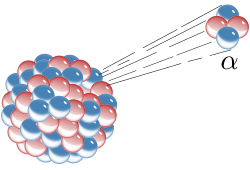
Alpha decay is a special process that happens in some types of atoms. An atom is like a tiny building block that makes up everything around us! 🏗️ When an atom undergoes alpha decay, it releases small particles called alpha particles. These particles are made of 2 protons and 2 neutrons, which are part of the atom's nucleus, or center. When an atom loses these particles, it transforms into a new kind of atom! This process was discovered over 100 years ago and helps scientists understand the world around us! 🌎
Gallery of Alpha Decay Facts For Kids
Alpha Decay In Nature
Alpha decay happens naturally in some elements, especially uranium and radium! 🌍These elements can be found in the ground, in rocks, and even in some minerals. As they decay, they change to other elements over time. Did you know that radon gas, which is radioactive, comes from the decay of uranium? 🏞️ Some places in the world have more radioactive elements than others, like in parts of Brazil and Canada. These natural processes show us how atoms change over time and how our planet works! 🌋
Discovery Of Alpha Decay
Alpha decay was discovered by a scientist named Ernest Rutherford in 1899. He was from New Zealand and made big discoveries about atoms! 🧑🔬 Rutherford used a special machine called a "Geiger counter" to detect the particles. He found that when uranium atoms changed, they released these tiny alpha particles! Uranium is a mineral found in many places, like Canada and Africa! Rutherford's work helped us learn that atoms can change and become different things over time. His discoveries are still studied and used today! 📚
Mechanism Of Alpha Decay
Alpha decay happens in a simple but amazing way! When an unstable atom has too much energy, it wants to become more stable. It's like a big balloon that has too much air! 🎈To let go of some energy, the atom shoots out an alpha particle, which is like a mini rocket! This leaves behind a new atom that has 2 fewer protons and 2 fewer neutrons. For example, when uranium-238 undergoes alpha decay, it turns into thorium-234! The new atom is different and more stable. Isn't that cool? 🌟
Applications Of Alpha Decay
Alpha decay is useful for several reasons! It helps scientists learn about radioactive materials and how they behave. 🧪This knowledge is used in medicine, like treating cancer! Doctors can use alpha particles to target and destroy cancer cells without harming too much of the healthy cells nearby. Alpha decay also helps us understand how the Earth and stars are made. Additionally, it plays a big role in creating electricity from nuclear power plants, which help to power our homes! 💡
Detection Of Alpha Particles
Detecting alpha particles is very important for scientists! These particles can be tricky because they can't travel very far; they can even be stopped by a piece of paper! 📄To find alpha particles, scientists use special detectors like Geiger counters and scintillation counters. The Geiger counter "clicks" when it detects alpha particles, helping scientists measure radiation levels. This is crucial in places where there might be radioactive materials, like old mines or nuclear power plants! 🏭Without these tools, it would be hard to keep everyone safe! 🔍
Future Research On Alpha Decay
Scientists are always learning more about alpha decay and its uses! 🤓They are researching ways to use alpha particles in medicine even more effectively, such as exploring new cancer therapies. Additionally, they are investigating how alpha decay affects the environment and helps to produce energy in nuclear reactors. 🌳By studying these processes, scientists hope to make our world a safer and healthier place! Who knows what amazing discoveries lie ahead? The more we learn about alpha decay, the more exciting things we can find out! 🚀
Health Effects Of Alpha Radiation
While alpha particles can be helpful in medicine, they can also be harmful! When alpha particles enter your body, they can damage cells, which might lead to health problems. 💔That's why it's important to stay away from radioactive materials! Alpha particles can't pass through skin, so if you keep radioactive substances outside your body, you're mostly safe. 🛑Scientists carefully study how alpha radiation affects our health to keep everyone safe, especially in places like hospitals or labs where it might be present! 👍
Alpha Decay Vs. Other Types Of Radiation
There are different ways atoms can release energy besides alpha decay! Two other mini–"decay" processes are beta decay and gamma decay. 🌀In beta decay, an atom spits out a tiny particle called a beta particle, which is smaller than an alpha particle! Gamma decay is a bit different; it releases high-energy rays instead of particles. 🌈Alpha particles are heavier than beta particles and can't travel as far. This makes alpha decay different and means that each type of radiation has unique properties and effects on matter. 🤓
Alpha Decay Facts For Kids Quiz


Make things. Learn new skills. Share safely.
DIY is a creative community where kids draw, build, explore ideas, and share.
No credit card required