Ethylene Facts For Kids
Ethylene is a colorless hydrocarbon gas with the formula C2H4, known for its role as a plant hormone and its extensive use in the chemical industry.

Set reading age
View for Kids
Easy to read and understand
View for Students
Clear, detailed explanations
View for Scholars
Deep dives and big ideas
Introduction
Ethylene is a special gas made of two carbon atoms and four hydrogen atoms! 🌱Its formula is C2H4, which means it’s a hydrocarbon. Ethylene is used in many ways, such as helping plants grow and making plastic. Think of it as a superhero for nature and industries! Ethylene is colorless and smells sweet, but it’s used very carefully. It can be found floating in the air we breathe, as it’s naturally made by plants and fruits. 🍏It’s also produced in factories to meet the needs of various industries around the world! 🌎
Gallery of Ethylene Facts For Kids
Ethylene In Industry
Ethylene is a superstar in the industry! 🌟It’s one of the most produced organic compounds in the world. Factories use it to create many products, like plastics and chemicals. 📦Ethylene is crucial for making polyethylene, which is a type of plastic used in shopping bags and containers. It’s also used in making antifreeze and high-quality synthetic rubber for tires. 🛞Around 150 million tons of ethylene are produced annually, mostly in countries like the USA, China, and Germany. This makes it super important for businesses and everyday life!
Properties Of Ethylene
Ethylene has some cool properties! 🌬️ It’s a gas at room temperature, and it’s lighter than air, which means it can float up. It doesn’t have a color, but it smells a little sweet. Some fun facts about ethylene are that it can burn if it meets fire! 🔥It also can dissolve in water, but not very well. Ethylene boils at around -104 degrees Celsius (-155 degrees Fahrenheit) — that sounds super cold! ❄️ These properties are important because they help scientists and farmers use ethylene properly to make things grow and create products!
Chemical Structure Of Ethylene
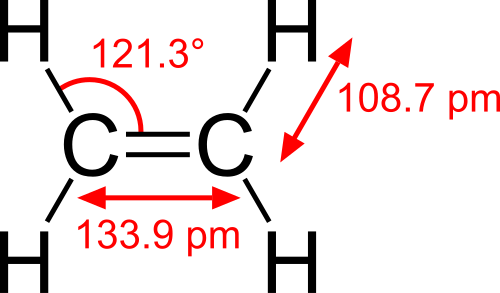
The structure of ethylene is quite interesting! 🧪It has a double bond between its two carbon atoms. This is shown as H2C=CH2, where the “=” means they are closely connected! Each carbon atom is also bonded to two hydrogen atoms. When we draw it, we can show the atoms as circles and the bonds as lines. The double bond makes ethylene very reactive, meaning it can easily change and combine with other chemicals! This structural design is what makes it so useful for creating different products, like plastics and fertilizers. 📈
Production Methods Of Ethylene
Ethylene is produced in different ways! 🏭One common method is called “cracking.” This is where bigger oil molecules are split into smaller ones, including ethylene. Another method involves using natural gas or biomass, like plants. Industrial plants can make tons of ethylene every year, around 150 million tons globally! 🌍These methods help meet the high demand for ethylene in industries like farming and making plastics. Safety is super important too, and factories need to follow special rules to make ethylene without harming the environment or workers! ⚙️
Safety And Environmental Impact
While ethylene is useful, it’s important to handle it safely! ⚠️ Ethylene can be flammable, which means it can catch fire easily, so special storage is needed. 🌡️ In factories, workers wear safety gear and follow rules to avoid accidents. Ethylene is made naturally by fruits and plants, which is safe! However, if too much is released into the environment, it can impact air quality. 🌈Scientists work to understand how to balance ethylene production so it remains helpful without harming nature or people! This way, we can enjoy its benefits while staying safe! 🕊️
Role Of Ethylene In Plant Biology
Did you know ethylene is a plant hormone? 🌿It plays an important role in helping plants grow! Ethylene helps plants respond to their environment, like when fruits ripen and flowers bloom. 🍃For example, when apples are picked, they naturally produce ethylene to ripen faster! It also helps plants deal with stress, such as stopping growth in response to bad weather or injury. Some plants even use ethylene to drop their leaves in autumn! 🍂So, ethylene is like a guiding friend for plants, helping them adapt to their surroundings!
Uses And Applications Of Ethylene
Ethylene is used in lots of surprising ways! 🌟Farmers use it to help fruits ripen, like bananas and tomatoes. 🍌🍅 This means when ethylene gas is released, it makes fruits change color and taste yummy! Ethylene also plays a big role in making plastics, such as bags, bottles, and toys. 🧸It’s even used in the production of antifreeze and some types of rubber! Besides that, ethylene helps make chemicals that are important for furniture and household items, making it a super useful gas in our everyday lives! 🏡
Detection And Measurement Of Ethylene
Detecting ethylene is super important for safety and science! 🔍Scientists use special devices called gas detectors to measure how much ethylene is in the air. These devices help make sure it’s at safe levels, especially in factories and farms. Ethylene can also be detected using chemical methods, like color-changing solutions that react when ethylene is present. 🌈In research, scientists measure ethylene to study how plants respond to it. This helps us understand plant health and growth better, making gardening and farming easier for everyone! 🌻
Historical Context Of Ethylene Discovery
The discovery of ethylene goes back to the 1800s! ⏳In 1805, a scientist named Michael Faraday discovered this gas while studying the heating of oils. Later, in the 19th century, chemists worked to understand ethylene more deeply. By 1866, Friedrich August Kekulé figured out its chemical structure, which helped scientists know it was a hydrocarbon. ⚗️ With time, ethylene became more known in agriculture, especially in helping fruits ripen. Today, it remains important in science and industry, helping us in ways that Michael Faraday couldn’t have imagined! 🌟
Ethylene Facts For Kids Quiz


Make things. Learn new skills. Share safely.
DIY is a creative community where kids draw, build, explore ideas, and share.
No credit card required