Carbonic Acid Facts For Kids
Carbonic acid is a chemical compound with the formula H2CO3, formed when carbon dioxide dissolves in water, playing essential roles in nature and industry.

Set reading age
View for Kids
Easy to read and understand
View for Students
Clear, detailed explanations
View for Scholars
Deep dives and big ideas
Introduction
Carbonic acid is a special substance made up of two hydrogen atoms, one carbon atom, and three oxygen atoms. Its chemical formula is H₂CO₃. 🌍This interesting acid is mostly found in dissolved carbon dioxide (CO₂) and is important for many living things! It forms when CO₂ mixes with water (H₂O). For example, when you blow bubbles in a glass of water, you create carbonic acid! This compound helps to keep our planet's atmosphere balanced and can be found in locations like oceans and rivers. 🌊
Gallery of Carbonic Acid Facts For Kids
Chemical Structure
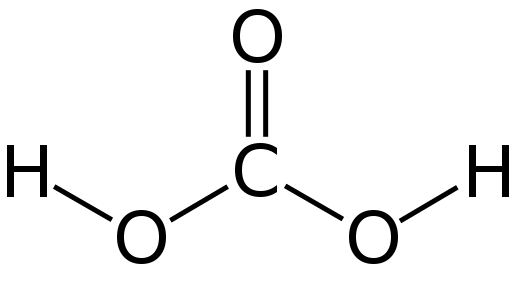
The chemical structure of carbonic acid looks like a fun building block! 💧Imagine two little balloons (the hydrogen atoms) holding onto a big beautiful ball (the carbon atom) that has three colorful strings (the oxygen atoms). This structure helps carbonic acid be a weak acid, which means it doesn't hurt us when we drink it. When it’s in water, carbonic acid can break apart (or dissociate) into its parts, releasing hydrogen ions (H⁺) and bicarbonate ions (HCO₃⁻). This is how it helps balance the pH of our bodies! ⚗️
Safety And Handling
Carbonic acid is very safe to use, especially in drinks and foods! 🥤Since it’s a weak acid, it doesn’t harm our bodies. However, like any substance, it should be treated with care. Always remember that if you find it in a strong form, like in laboratories, only adults should handle it, and they use special safety gear. 🤓In the kitchen, it’s perfectly safe to enjoy carbonated beverages, but it's important to enjoy them in moderation and maintain a balanced diet. Stay safe and fizzy! 🎉
Environmental Impact
Carbonic acid is crucial for our planet’s health and climate! 🌍It helps regulate the pH level of oceans, making sure sea creatures like corals and shellfish can thrive. However, too much carbon dioxide released by cars and factories is causing more carbonic acid to form, which can harm marine life! 🐠This is known as ocean acidification. It can make it difficult for shellfish to grow their shells and affect coral reefs. By taking care of our environment and reducing pollution, we can help maintain a healthy balance of carbonic acid in our oceans! 🌊
Formation And Reactions
Carbonic acid is formed when carbon dioxide (CO₂) mixes with water (H₂O) in a process called hydration. 🌧️ When you open a soda can, CO₂ escapes and some of it combines with the water in the soda to create carbonic acid. The reaction looks like this: CO₂ + H₂O ⇌ H₂CO₃. The carbonic acid can then break apart into bicarbonate (HCO₃⁻) and hydrogen ions (H⁺), which is great for our bodies because it helps with breathing and digestion. So, every time we drink fizzy drinks, we get a little taste of carbonic acid! 🥤
Historical Significance
Carbonic acid has been important for thousands of years, dating back to Ancient Rome, where sparkling water was first bottled! 🏺People liked fizzy drinks, and they were a special treat. Scientists discovered more about carbonic acid in the 18th century when chemists like Joseph Priestley started experimenting with CO₂ and water. This helped us learn how carbonic acid affects our health and the environment today. Because of these discoveries, we now understand how important this compound is in our lives, drinks, and ecosystems! 📚
Applications In Industry
Carbonic acid is very helpful and is used in many industries! 🏭Firstly, it’s used in beverages like sodas, sparkling water, and beer, making them bubbly and delicious. It helps preserve food and keep it fresh too! 🍓Secondly, carbonic acid can be found in special cleaning products and even in the production of some chemicals and medicines. Thirdly, it’s important in agriculture; farmers use it to make soil more balanced and healthy for plants. So, carbonic acid isn’t just important; it helps make everyday things even better! 🌾
Carbonic Acid In Beverages
You might have noticed that bubbles make drinks exciting, and that’s because of carbonic acid! 🍹When carbon dioxide is added to liquids like soda, it creates carbonic acid, giving it a fizzy taste. The bubbles pop when you drink the soda, making it feel refreshing! Drinks like sparkling water and tonic water also contain carbonic acid, which helps keep them bubbly. When you drink, it adds a fizzy sensation that many people love! So, every time you sip that fizzy drink, you’re enjoying a little bit of science! ✨
Role In Biological Systems
Carbonic acid plays a big role in our bodies and the environment! 🌱It helps control the acidity in our bloodstream, which is important for carrying oxygen to all parts of our body. When we breathe out carbon dioxide, it can turn into carbonic acid in our blood, keeping our pH balanced! It's also important for forming bicarbonate, which helps our bodies stay healthy. Not just in our bodies, it also helps fish and other sea creatures survive in oceans, maintaining a healthy ecosystem. So, carbonic acid is like a superhero for life! 🦸♀️
Properties Of Carbonic Acid
Carbonic acid has some cool properties that make it unique! 🌟It’s a weak acid, which means it doesn’t taste very sour like lemon juice. It helps keep our blood healthy by balancing acidity. Carbonic acid exists mainly in water and can only be found in small amounts since it breaks down quickly into carbon dioxide and water. It has a colorless appearance and is not very reactive, making it safe for our bodies! This compound helps form a protective boundary in our cells and is essential for living beings! 🩺
Carbonic Acid Facts For Kids Quiz


Make things. Learn new skills. Share safely.
DIY is a creative community where kids draw, build, explore ideas, and share.
No credit card required